Our Scientific Approach

Our scientific approach:
targeting the bradykinin B2 receptor
Hereditary angioedema (HAE) most commonly occurs as a result of insufficient levels or function of a protein called C1 inhibitor (C1-INH), a naturally occurring inhibitor of the plasma kallikrein enzyme. C1-INH deficiency results in uncontrolled plasma kallikrein activity which leads to elevated levels of bradykinin and painful swelling affecting different body areas.
Antagonism of the bradykinin B2 receptor is a well-tolerated, well-established, clinically proven mechanism of action in HAE.1,2,3 Based on detailed insights into the three-dimensional structure of the B2 receptor, Pharvaris designed a small molecule that fits in the pocket of the B2 receptor, where bradykinin naturally binds.4
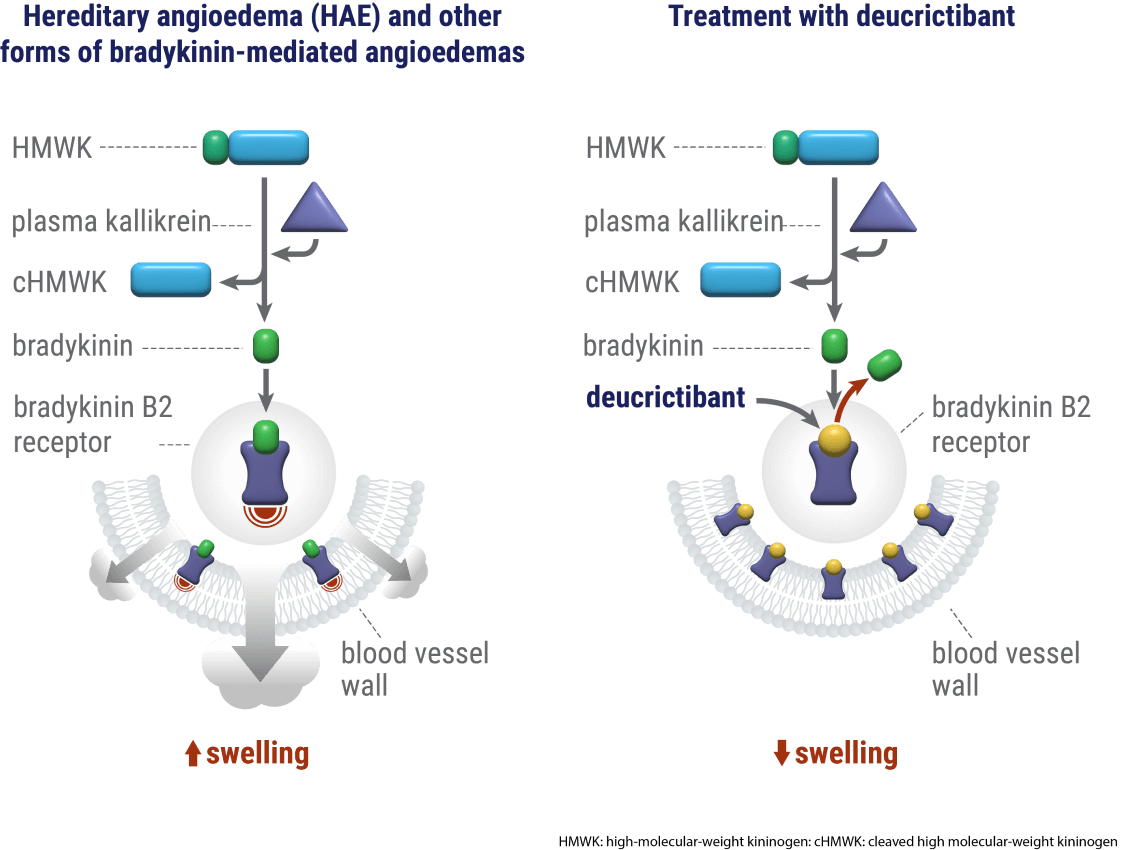
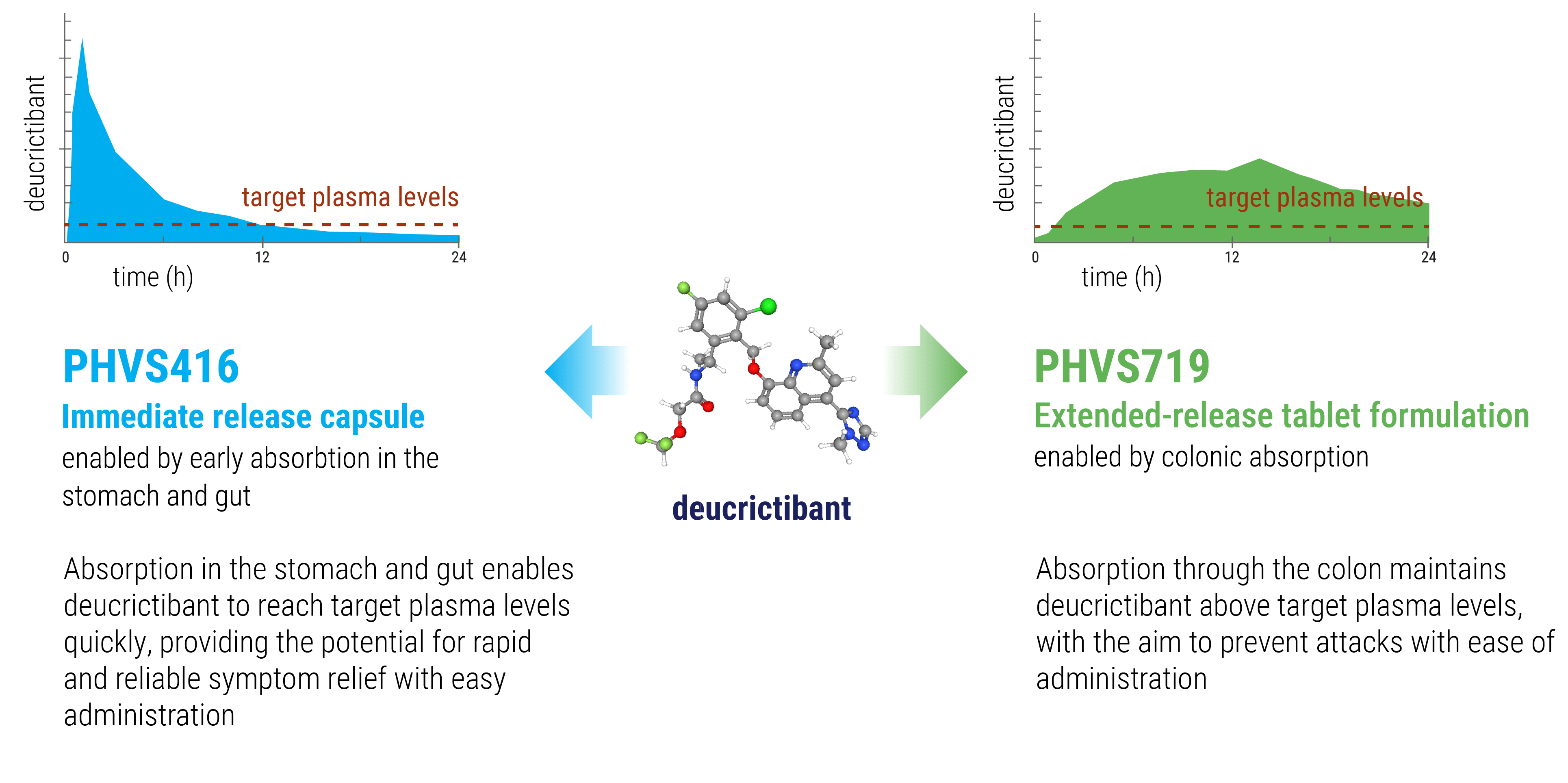
By inhibiting bradykinin signaling through the bradykinin B2 receptor, deucrictibant has the potential to treat the clinical manifestations of an HAE attack and also to prevent the occurrence of attacks. The therapeutic capabilities of deucrictibant can be harnessed in two distinct oral formulations: an immediate-release capsule to enable rapid onset of activity for the acute treatment of HAE attacks, and an extended-release tablet to enable sustained efficacy in the prophylactic treatment of HAE. With these two formulations, we aspire to provide people living with HAE treatment options with robust efficacy, a well-tolerated profile & low-burden administration which can be taken anywhere, anytime.
Publications
Pharmacological profile of PHA121 published in International Pharmacology
In Vitro Pharmacological Profile of a New Small Molecule Bradykinin B2 Receptor Antagonist
Posters and Presentations
Efficacy and Safety of Oral Deucrictibant, a Bradykinin B2 Receptor Antagonist, in Prophylaxis of Hereditary Angioedema Attacks: Results of CHAPTER-1 Phase 2 Trial
Relief and Resolution of Attack Symptoms Following On-Demand Treatment With a Single Dose of Oral Bradykinin B2 Receptor Antagonist Deucrictibant Immediate-Release Capsule in Patients With Hereditary Angioedema
1 Cicardi M et al. N Engl J Med 2010;363:532-41
2 Lumry WR et al. Ann Allergy Asthma Immunol 2011;107:529-37
3 Maurer M et al. Clin Exp Allergy 2022;52:1048-58
4 Lesage A, et al. In Vitro Pharmacological Profile of a New Small Molecule Bradykinin B2 Receptor Antagonist. Front Pharmacol 2020;11:916.